How dangerous are serrated adenomas?
Thomas Rösch, Hamburg
Gut. 2015 Mar 2. pii: gutjnl-2014-308603 und Gut. 2014 Nov 16. pii: gutjnl-2014-307793
| Clinical and endoscopic predictors of cytological dysplasia or cancer in a prospective multicentre study of large sessile serrated adenomas/polyps |
| Nicholas G Burgess, Maria Pellise, Kavinderjit S Nanda, Luke F Hourigan, Simon A Zanati, Gregor J Brown, Rajvinder Singh, Stephen J Williams, Spiro C Raftopoulos, Donald Ormonde, Alan Moss, Karen Byth, Heok P’Ng, Duncan McLeod, Michael J Bourke |
Objective
The serrated neoplasia pathway accounts for up to 30% of all sporadic colorectal cancers (CRCs). Sessile serrated adenomas/polyps (SSA/Ps) with cytological dysplasia (SSA/P-D) are a high-risk serrated CRC precursor with little existing data. We aimed to describe the clinical and endoscopic predictors of SSA/PD and high grade dysplasia (HGD) or cancer.
Design
Prospective multicentre data of SSA/Ps ≥20 mm referred for treatment by endoscopic mucosal resection (September 2008–July 2013) were analysed. Imaging and lesion assessment was standardised. Histological findings were correlated with clinical and endoscopic findings.
Results
268 SSA/Ps were found in 207/1546 patients (13.4%). SSA/P-D comprised 32.4% of SSA/Ps ≥20 mm. Cancer occurred in 3.9%. On multivariable analysis, SSA/P-D was associated with increasing age (OR=1.69 per decade; 95% CI (1.19 to 2.40), p0.004) and increasing lesion size (OR=1.90 per 10 mm; 95% CI (1.30 to 2.78), p0.001), an ‘adenomatous’ pit pattern (Kudo III, IV or V) (OR=3.98; 95% CI (1.94 to 8.15), p<0.001) and any 0-Is component within a SSA/P (OR=3.10; 95% CI (1.19 to 8.12) p0.021). Conventional type dysplasia was more likely to exhibit an adenomatous pit pattern than serrated dysplasia. HGD or cancer was present in 7.2% and on multivariable analysis, was associated with increasing age (OR=2.0 per decade; 95% CI 1.13 to 3.56) p0.017) and any Paris 0-Is component (OR=10.2; 95% CI 3.18 to 32.4, p<0.001).
Conclusions
Simple assessment tools allow endoscopists to predict SSA/P-D or HGD/cancer in SSA/Ps ≥20 mm. Correct prediction is limited by failure to recognise SSA/P-D which may mimic conventional adenoma. Understanding the concept of SSA/P-D and the pitfalls of SSA/P assessment may improve detection, recognition and resection and potentially reduce interval cancer.
| Long-term risk of colorectal cancer in individuals with serrated polyps |
| Øyvind Holme, Michael Bretthauer, Tor J Eide, Else Marit Løberg, Krzysztof Grzyb, Magnus Løberg, Mette Kalager, Hans-Olov Adami, Øystein Kjellevold, Geir Hoff |
Objective
Although serrated polyps may be precursors of colorectal cancer (CRC), prospective data on the longterm CRC risk in individuals with serrated polyps are lacking.
Design
In a population-based randomised trial, 12 955 individuals aged 50–64 years were screened with flexible sigmoidoscopy, while 78 220 individuals comprised the control arm. We used Cox models to estimate HRs with 95% CIs for CRC among individuals with ≥1 large serrated polyp (≥10 mm in diameter), compared with individuals with adenomas at screening, and to population controls, and multivariate logistic regression to assess polyp risk factors for CRC.
Results
A total of 103 individuals had large serrated polyps, of which 81 were included in the analyses. Nonadvanced adenomas were found in 1488 individuals, advanced adenomas in 701. Median follow-up was 10.9 years. Compared with the control arm, the HR for CRC was 2.5 (95% CI 0.8 to 7.8) in individuals with large serrated polyps, 2.0 (95% CI 1.3 to 2.9) in individuals with advanced adenomas and 0.6 (95% CI 0.4 to 1.1) in individuals with non-advanced adenomas. A large serrated polyp was an independent risk factor for CRC, adjusted for histology, size and multiplicity of
concomitant adenomas (OR 3.3; 95% CI 1.3 to 8.6). Twenty-three large serrated polyps found at screening were left in situ for a median of 11.0 years. None developed into a malignant tumour.
Conclusions
Individuals with large serrated polyps have an increased risk of CRC, comparable with
individuals with advanced adenomas. However, this risk may not be related to malignant growth of the serrated polyp.
What you need to know
Sessile serrated adenomas (SSAs) are the latest fashion in gastroenterological endoscopy. Individual histopathological examinations have shown that there is a separate pathway to carcinoma (1, 2), apparently also with individual case reports of very aggressive carcinomas (3, 4). The prevalence data for SSAs range from 2.8% to 9%, depending on the specialization of the centers and the indication involved (5–7). However, the definitions used are unclear, and the term “sessile serrated adenoma/polyp” (SSA-P) is therefore often used synonymously, as the histological distinction from a “genuine” hyperplastic polyp (HP), evidently with no recognizable risk of malignancy, is difficult. There is a high rate of diagnostic switching from HP to SSA when experts review histological findings (8, 9), but with wide differences of opinion (interobserver variability) among the experts (10–12).
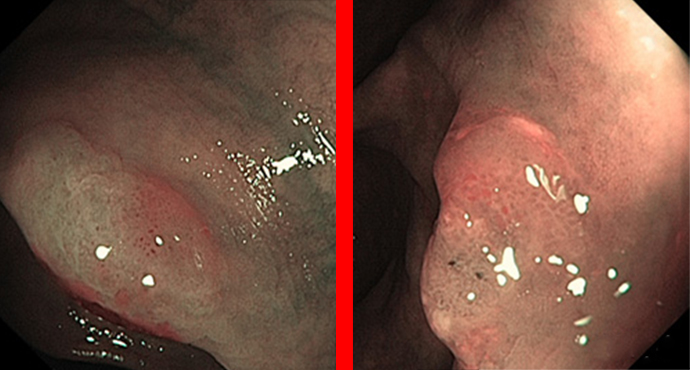
As shallow, often poorly visible lesions mainly located on the right side, SSAs are regarded as one of the main culprits behind interval carcinomas. This might be due on the one hand to the fact that these lesions are characteristically very shallow, often have a “translucent” appearance, and are often covered with yellowish mucus that looks like residual soiling. On the other hand, it is also claimed that the lesions are biologically more aggressive (3, 4).
Among the few larger and clinically oriented series that have been published, two relevant papers have recently been published in the journal Gut. They both show that the lesions are associated with a certain amount of risk, but that the risk is quite similar to that of conventional adenomas.
The analysis of 268 serrated adenomas in the large Australian adenoma study showed a rate of cytological dysplasia amounting to around one-third. Very similar endoscopic criteria as those used for conventional (shallow) adenomas — namely, age, size of lesion, and adenoma-like appearance — were found to have a certain amount of predictive value.
The presence of cytological “dysplasia” corresponds approximately to the grade of dysplasia (low-grade / high-grade intraepithelial neoplasia) with conventional adenomas; without this type of dysplasia, SSA-Ps then tend to resemble hyperplasias more. In SSA-Ps that are 2 cm in size or larger, high-grade dysplasias are found in around 7% of cases, a rate similar to that with conventional adenomas. A characteristic finding here was increasing age among the patients and also sessile (raised) portions — also similar to shallow adenomas. In very simplified terms, therefore: the more that SSA-Ps look like adenomas, the more dangerous they are.
The other analysis, from the large Norwegian sigmoidoscopy study, presented long-term data with a 10-year follow-up for 81 patients with larger (≥ 1 cm) serrated lesions. Relative to the patients, the carcinoma risk was as high as for advanced adenomas. However, 23 of the lesions were left in situ, and after 11 years no carcinoma had arisen directly from any of them.
Methodologically, the study is a subanalysis of a large sigmoidoscopy study (13) — i.e., patients in whom polyps were found on sigmoidoscopy then underwent colonoscopy; the serrated polyps found were thus derived from both examinations. Patients with negative findings in the distal colon and SSA-Ps proximal to the range of penetration of sigmoidoscopy were thus of course excluded — potentially a significant limitation. Despite this, the study is an attractive one, with its sensationally long follow-up period of nearly 11 years. Eighty-one out of 103 patients with SSA-Ps that were 1 cm or larger were included. During the follow-up period, there was a similarly high risk of carcinoma (hazard ratio 2.5) as with advanced adenomas (HR 2.0). From the parallel findings for 23 patients with larger SSA-Ps that were left in situ without carcinoma developing in even a single case over the mean 11-year period, the authors conclude that the patients were at high risk, like others who have polyps, but that it is not inevitable that cancer will develop from the SSA-Ps themselves. The limited numbers of cases and possibly outdated histological criteria are of course possible limitations in this retrospective analysis.
Both of these two studies are highly interesting and do not offer any evidence that serrated lesions are any more dangerous than conventional (shallow) adenomas in everyday clinical practice. It is not yet clear whether they are more easily overlooked than adenomas.
A careful search for sessile serrated adenomas should certainly still be carried out in everyday clinical work, but particularly after these two important papers, the findings should not be overrated — e.g., by setting shorter follow-up intervals. Current guideline discussions are tending towards assigning similar follow-up intervals to SSA-Ps as to adenomas. Their histopathological variability would still have to be clarified, however, as a “gold standard.”
References
- Rex DK, Ahnen DJ, Baron JA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol 2012;107:1315-29; quiz 1314, 1330.
- Rosty C, Hewett DG, Brown IS, et al. Serrated polyps of the large intestine: current understanding of diagnosis, pathogenesis, and clinical management. J Gastroenterol 2013;48:287-302.
- Kriegl L, Neumann J, Vieth M, et al. Up and downregulation of p16(Ink4a) expression in BRAF-mutated polyps/adenomas indicates a senescence barrier in the serrated route to colon cancer. Mod Pathol 2011;24:1015-22.
- Kriegl L, Vieth M, Kirchner T, et al. Up-regulation of c-MYC and SIRT1 expression correlates with malignant transformation in the serrated route to colorectal cancer. Oncotarget 2012;3:1182-93.
- pring KJ, Zhao ZZ, Karamatic R, et al. High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology 2006;131:1400-7.
- Kahi CJ, Li X, Eckert GJ, et al. High colonoscopic prevalence of proximal colon serrated polyps in average-risk men and women. Gastrointest Endosc 2012;75:515-20.
- Hetzel JT, Huang CS, Coukos JA, et al. Variation in the detection of serrated polyps in an average risk colorectal cancer screening cohort. Am J Gastroenterol 2010;105:2656-64.
- Khalid O, Radaideh S, Cummings OW, et al. Reinterpretation of histology of proximal colon polyps called hyperplastic in 2001. World J Gastroenterol 2009;15:3767-70.
- Singh H, Bay D, Ip S, et al. Pathological reassessment of hyperplastic colon polyps in a city-wide pathology practice: implications for polyp surveillance recommendations. Gastrointest Endosc 2012;76:1003-8.
- Wong NA, Hunt LP, Novelli MR, et al. Observer agreement in the diagnosis of serrated polyps of the large bowel. Histopathology 2009;55:63-6.
- Ensari A, Bilezikci B, Carneiro F, et al. Serrated polyps of the colon: how reproducible is their classification? Virchows Arch 2012;461:495-504.
- Glatz K, Pritt B, Glatz D, et al. A multinational, internet-based assessment of observer variability in the diagnosis of serrated colorectal polyps. Am J Clin Pathol 2007;127:938-45.
- Holme O, Loberg M, Kalager M, et al. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: a randomized clinical trial. Jama 2014;312:606-15.