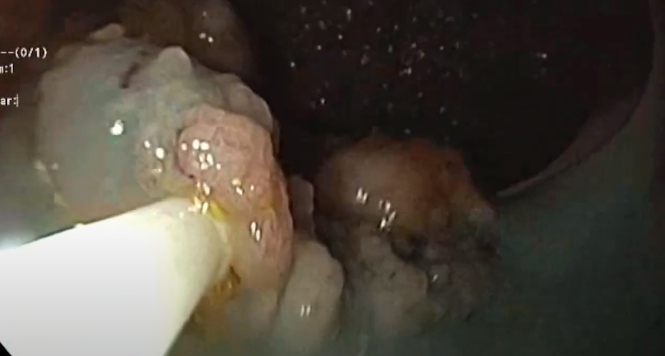
ORISE Associated With Submucosal Fullness and Distortion at Follow-Up EMR
Douglas K. Rex, MD, MASGE, reviewing Lahr RE, et al. Gastrointest Endosc 2022 May 9.
ORISE (Boston Scientific, Marlborough, Mass, USA) is a viscous gel for submucosal injection. When ORISE is used for endoscopic mucosal resection (EMR) or endoscopic submucosal dissection, histologic specimens may show basophilic material that resembles mucin on hematoxylin eosin stains. Biopsies or surgical resections performed at a later date show the injected material appears eosinophilic and to be associated with a multinucleated giant cell reaction.
In this study, an endoscopist performing EMRs with ORISE noted that the submucosa often appeared full, nodular, or otherwise distorted at the first surveillance follow-up. To evaluate this finding, two endoscopic experts blindly assessed 30 first-surveillance EMR scars after ORISE use and 30 after injection of other agents. Although the kappa value for agreement in submucosal distortion scores between the two experts showed minimal agreement, both experts scored the submucosal distortion as significantly greater in ORISE cases than in cases in which other materials were used.

COMMENTThe authors noted that submucosal distortion in these cases with ORISE produced no difficulty in differentiating recurrent adenomas from no recurrence. Further, some of the poor agreement in submucosal distortion scores may have been caused by mucosal clip artifact, which was present in a number of cases. The submucosal distortion appears completely benign but should be recognized by endoscopists to avoid misguided concern that the submucosal nodules or mounds represent submucosal tumor growth.
Note to readers: At the time we reviewed this paper, its publisher noted that it was not in final form and that subsequent changes might be made.
CITATION(S)
Lahr RE, DeWitt JM, Zhang D, Rex DK. Assessment of submucosal distortion and mass effect seen at follow-up after colorectal endoscopic mucosal resection with ORISE. Gastrointest Endosc 2022 May 9. (Epub ahead of print) (https://doi.org/10.1016/j.gie.2022.04.1344)